Natural pH Level for Hair
A: Well, the main reason most salons claim that their products are better for the hair is a matter of pH levels. The pH (potential hydrogen) scale is a measure of the acidity or alkalinity of a substance. Here's a quick and simple explanation of pH and how it works.
In pure water, some of the water molecules naturally ionize into hydrogen ions and hydroxide ions. The pH scale measures these ions. The hydrogen ion (H+) is acidic, while the hydroxide ion (OH) is alkaline. pH is only possible because of the ionization of water. In pure water, every water molecule produces one hydrogen ion and one hydroxide ion. Pure water has a neutral pH because it has an even number of hydrogen and hydroxide ions.
The pH scale is the measure of relative alkalinity (number of hydroxide ions) or acidity (number of hydrogen ions) in a substance. “pH” as a term originates from the French term ‘pouvoir hydrogene’ (or hydrogen power). The “p” is written lower case to represent a quantity while the “H” is capitalized because it represents an element, therefore “pH” is the measure of the quantity of an element in a substance (in this case the amount of Hydrogen).
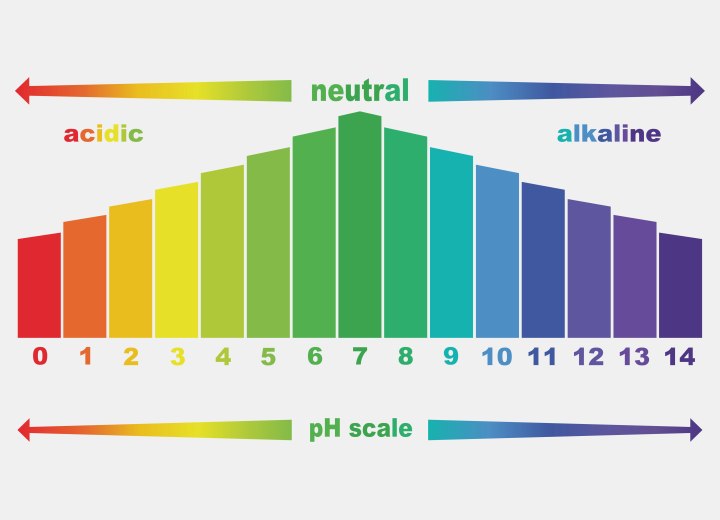
pH is measured on a scale of 0-14, with 7 being a neutral solution. Each step on the scale is a tenfold increase in alkalinity (moving up the scale) or in acidity (moving down the scale). For example: skin and hair have an average pH of 5, which means that pure water is 100 times more alkaline than skin or hair, and skin or hair is 100 times more acidic than pure water.
Any substance with a pH that is greater than 7.0 is considered an alkali, and any substance with a pH lower than 7.0 is considered an acid. Acids owe their chemical reactivity to the presence of Hydrogen ions (H+), taste sour, and will turn litmus paper from blue to red. Acids affect the hair by contracting and hardening it.
Alkalis owe their chemical reactivity to the hydroxide ion (OH). The terms "alkali" and "base" are interchangeable. Alkalis have a bitter taste, turn litmus paper from red to blue, and feel soapy and slippery on the skin. They affect the hair by softening and swelling it.
When acids and alkalis are combined in equal portions, they neutralize one another.
When it comes to salon products and their pH levels, most salons claim that their products are better for the hair because they are gentler (more closely match the natural pH of hair). This could be tested by using litmus paper to gauge the pH level of these salon products, and comparing the pH levels with the established natural level of 5 for hair. (Hair and skin actually have a pH that ranges from 4.5 to 5.5, and is typically averaged to 5.0.) You could test a number of salon products (shampoos and conditioners) and a number of store brand versions.
©Hairfinder.com
See also: Are the hair products that you can buy in hair salons really better?